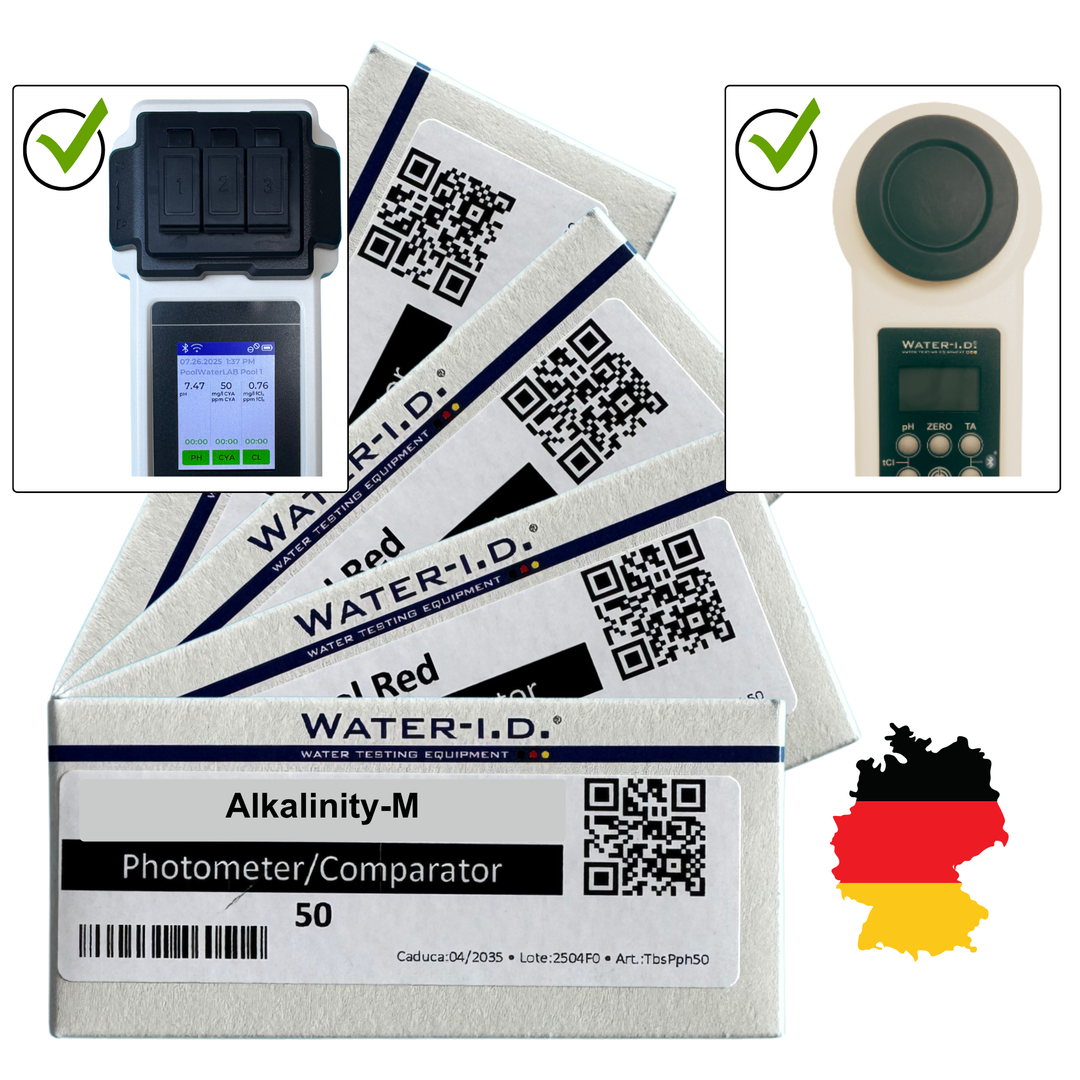
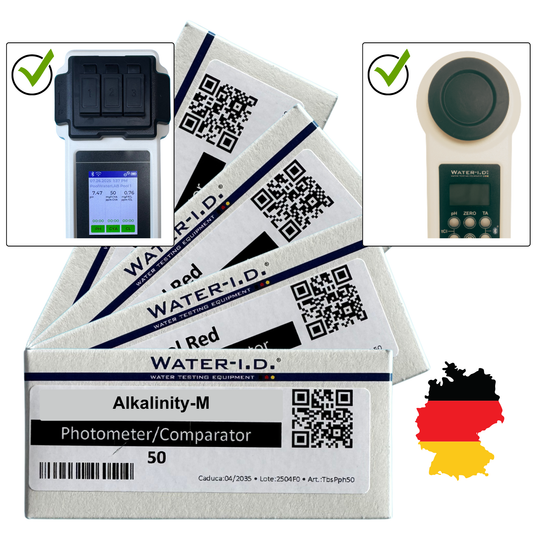
Alkalinity-M Reagents – for Testing Alkalinity (50 Tablets) for Poollab 1.0, 2.0 & PoolWaterLAB
- Developed in Germany for the US market
- Free return within 30 days
- Item is located in the USA and ships from the USA. Orders ship within 24 hours. Free shipping on orders over $100. To find out the cost of worldwide shipping, simply add items to your cart and enter your shipping details — the rate will be calculated automatically.
- In stock, ready to ship
- Backordered, shipping soon
Acid capacity (alkalinity) | 0.0 - 200 mg/l (ppm) | reagent for measurements - Alkalinity-M. Shelf life tablet reagents 2 years. ( TbsPTA50 )
KS4,3 Acidity is also known as m-Alkalinity, Total Alkalinity, Hydrogen Carbonate Hardness, Acid Buffering Power, Temporary Hardness, … Alkalinity describes the ability of water to buffer the increase of ph value influencing chemicals (flocculants, disinfection media – e.g. chlorine products – lowering or raising pH). To provide a sufficient buffering effect, alkalinity should amount to at least 0.7 mol/m3 and/or mmol/l. This value represents the hydrogen carbonated materials dissolved in water. The buffering effect in the 4.2 – 8.2 pH range relies on a balance between hydrogen carbonate ions and carbon dioxide dissolved in water. Should chemicals that lower the pH value of water be added (acids), then the hydrogen carbonate ion combines with these to form carbonic acid (which in turn dissolves into carbon dioxide and water) and water. At a 4.3 pH value all hydrogen carbonate ions are depleted; thus the KS4,3 Acidity designation. Should in contrast chemicals be added that raise the pH value (bases), then hydrogen carbonate ions form again out of dissolved carbon dioxide and water. The modified relationship between dissolved carbon dioxide and hydrogen carbonate ions thus determines a new pH value. The buffering capacity of water becomes too low at alkalinities below 0.7 mmol/l, thus making it difficult to determine the pH value. In such cases small amounts of acids and bases will immediately and intensively change the pH value. Furthermore, water will have a corrosive effect on pipe mains. An alkalinity value which is too low can be increased through the addition of sodium hydrogen carbonate and/or sodium carbonate. When alkalinity values are high, however, the buffering effect is too large and large amounts of pH regulators are needed in order to achieve a change in pH. Additionally, when conditions are unfavorable (warming, pH > 8.2), calcium tends to precipitate because carbonate ions form out of hydrogen carbonate ions which in turn form water-insoluble compounds in the presence of calcium or magnesium (see Total Hardness). Alkalinity that is too high can be corrected through – at least partial – replacement of water. Because pH values above 8.2 will stop the equilibrium between hydrogen carbonate ions and carbonate ions, the alkalinity of the water must then (pH value over 8.2) be measured with the Alkalinity-P method.